What Is A Disadvantage Of Using A Space-filling Model To Show A Chemical Compound?

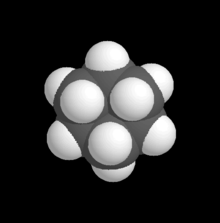
A space-filling model of due north-octane, the straight chain (normal) hydrocarbon composed of 8 carbons and xviii hydrogens, formulae: CH3CH2(CH2)ivCH2CH3 or C
8 H
18 . Notation, the representative shown is of a single conformational "pose" of a population of molecules, which, because of low Gibbs energy barriers to rotation near its carbon-carbon bonds (giving the carbon "chain" smashing flexibility), normally is composed of a very large number of dissimilar such conformations (e.g., in solution).

An example of a 3-dimensional, infinite-filling model of a complex molecule, THC, the active amanuensis in marijuana.
In chemical science, a space-filling model, also known as a calotte model, is a type of three-dimensional (3D) molecular model where the atoms are represented past spheres whose radii are proportional to the radii of the atoms and whose center-to-centre distances are proportional to the distances between the diminutive nuclei, all in the aforementioned scale. Atoms of unlike chemical elements are ordinarily represented by spheres of different colors.
Space-filling calotte models are also referred to as CPK models afterwards the chemists Robert Corey, Linus Pauling, and Walter Koltun, who over a span of time adult the modeling concept into a useful form.[ane] They are distinguished from other 3D representations, such every bit the ball-and-stick and skeletal models, by the use of the "total size" space-filling spheres for the atoms. They are useful for visualizing the effective shape and relative dimensions of the molecule, and the shapes of surface a given static conformer might present. On the other hand, these models mask the chemical bonds betwixt the atoms, and make it hard to encounter the construction of the molecule that is obscured past the atoms nearest to the viewer in a detail pose. For this reason, such models are of greater utility if they can exist used dynamically, especially when used with circuitous molecules (e.g., see the greater understanding of the molecules shape given when the THC model is clicked on to rotate).
History [edit]
Infinite-filling models arise out of a want to represent molecules in ways that reflect the electronic surfaces that molecules present, that dictate how they interact, one with another (or with surfaces, or macromolecules such every bit enzymes, etc.). Crystallographic data are the starting point for understanding static molecular structure, and these data incorporate the information rigorously required to generate space-filling representations (e.g., encounter these crystallographic models); near often, yet, crystallographers nowadays the locations of atoms derived from crystallography via "thermal ellipsoids" whose cut-off parameters are set for convenience both to show the cantlet locations (with anisotropies), and to allow representation of the covalent bonds or other interactions betwixt atoms as lines. In brusk, for reasons of utility, crystallographic data historically accept appeared in presentations closer to ball-and-stick models. Hence, while crystallographic data contain the information to create space-filling models, it remained for individuals interested in modeling an effective static shape of a molecule, and the space information technology occupied, and the ways in which information technology might present a surface to another molecule, to develop the ceremonial shown above.
In 1952, Robert Corey and Linus Pauling described authentic scale models of molecules which they had built at Caltech.[ane] In their models, they envisioned the surface of the molecule equally existence adamant by the van der Waals radius of each atom of the molecule, and crafted atoms every bit hardwood spheres of diameter proportional to each atom'due south van der Waals radius, in the scale 1 inch = 1 Å. To allow bonds between atoms a portion of each sphere was cutting abroad to create a pair of matching flat faces, with the cuts dimensioned so that the distance between sphere centers was proportional to the lengths of standard types of chemical bonds.[one] A connector was designed—a metal bushing that threaded into each sphere at the center of each apartment face. The two spheres were then firmly held together by a metal rod inserted into the pair of opposing bushing (with fastening past screws). The models too had special features to allow representation of hydrogen bonds.[i] [ verification needed ] [2]

An instance of a 3D, space-filling model of a uncomplicated molecule, sulfur dioxide, Then2, showing the electrostatic potential surface, computed for the molecule using the Spartan software suite of computational chemistry tools. It is shaded from blue for electropositive areas to red for electronegative areas. The surface was generated past calculating the energy of interaction of a spherical point positive charge (e.g., a proton, H+,) with the molecule's atoms and bonding electrons, in a series of detached computational steps. Here, the electrostatic surface emphasizes the electron deficiency of the sulfur atom, suggesting interactions in which it might engage, and chemical reactions information technology might undergo.

An example of a 3D, space-filling model of a very complex macromolecule, a protein, the prison cell membrane-spanning β2 adrenoreceptor, a Thousand protein-coupled receptor, in this prototype, viewed as if looking downwardly onto the extracellular surface. The electrostatic potential surface was applied to a model with atom positions determined by crystallography (PDB code 2RH1); the electrostatic surface was computed using Adaptive Poisson-Boltzmann Solver (APBS) freeware.[3] It is again shaded bluish for electropositive areas to ruddy for electronegative areas. Somewhat credible, in stick representation in yellow, red and blueish, in a groove at the top of the receptor, is a minor molecule ligand bound to it, the amanuensis carazolol, a fractional inverse agonist which, through this bounden, antagonizes binding of the normal ligand, the neurotransmitter/hormone epinephrine. In response to bounden epinephrine, this receptor, in conjunction with an 50-type calcium channel, mediates physiologic responses such as smooth muscle relaxation and bronchodilation. All of such binding interactions and the function of the receptor in signal transduction are mediated by electrostatic effects, and in mod structure work they are often studied using similar space filling models.
In 1965, Walter L. Koltun designed and patented a simplified arrangement with molded plastic atoms of various colours, which were joined past specially designed snap connectors; this simpler system accomplished essentially the aforementioned ends as the Corey-Pauling arrangement,[4] [ not-principal source needed ] [ improve source needed ] and allowed for the evolution of the models every bit a popular way of working with molecules in training and research environments. Such color-coded, bail length-defined, van der Waal'due south-type space-filling models are now usually known every bit CPK models, after these 3 developers of the specific concept.
In modernistic inquiry efforts, attention returned to utilise of information-rich crystallographic models in combination with traditional and new computational methods to provide infinite-filling models of molecules, both uncomplicated and complex, where added information such as which portions of the surface of the molecule were readily attainable to solvent, or how the electrostatic characteristics of a space-filling representation—which in the CPK case is almost fully left to the imagination—could be added to the visual models created. The two closing images requite examples of the latter type of adding and representation, and its utility.
See also [edit]
- Ball-and-stick model
- Van der Waals surface
- CPK coloring
- Molecular graphics
- Software for molecular modeling
- Molecular design software
References [edit]
- ^ a b c d Corey, Robert B.; Pauling, Linus (1953). "Molecular models of amino acids, peptides, and proteins" (PDF). Review of Scientific Instruments. 8 (24): 621–627. Bibcode:1953RScI...24..621C. doi:ten.1063/one.1770803. Retrieved ix March 2020.
- ^ In the aforementioned paper Corey and Pauling also briefly describe a much simpler but less accurate type of model, with prophylactic-similar polyvinyl plastic spheres in the scale 1 inch = 2Å and connected by snap fasteners. See Corey & Pauling, 1953, op. cit.
- ^ Baker, Northward.A., Sept, D., Joseph, S., Holst, Thou.J. & McCammon, J.A., 2001, "Electrostatics of nanosystems: Application to microtubules and the ribosome," Proc. Natl. Acad. Sci. U.Southward.A. 98: pp. 10037-10041, encounter [i], and "Archived copy". Archived from the original on 2015-06-24. Retrieved 2015-06-23 .
{{cite spider web}}: CS1 maint: archived copy every bit title (link), and [2], accessed 23 June 2015. - ^ Walter L. Koltun (1965), Infinite filling atomic units and connectors for molecular models. U. S. Patent 3170246.[ not-master source needed ] [ amend source needed ]
External links [edit]
- More than on molecular models and a couple of examples from chemistry and biology (article is in High german)
Gallery [edit]
What Is A Disadvantage Of Using A Space-filling Model To Show A Chemical Compound?,
Source: https://en.wikipedia.org/wiki/Space-filling_model
Posted by: scottwhounces1938.blogspot.com


0 Response to "What Is A Disadvantage Of Using A Space-filling Model To Show A Chemical Compound?"
Post a Comment